The AVENIO Tumor Tissue CGP Kit is an in-house next-generation sequencing (NGS) research assay that provides comprehensive genomic profiling of solid tumors from formalin-fixed paraffin-embedded (FFPE) tissue samples.

The AVENIO Tumor Tissue CGP Kit is an in-house next-generation sequencing (NGS) research assay that provides comprehensive genomic profiling of solid tumors from formalin-fixed paraffin-embedded (FFPE) tissue samples.
The AVENIO Tumor Tissue CGP Kit is an in-house next-generation sequencing (NGS) research assay that provides comprehensive genomic profiling of solid tumors from formalin-fixed paraffin-embedded (FFPE) tissue samples. Designed to match the content of the 324 gene FoundationOne® CDx panel, the kit leverages the proven technology of Foundation Medicine and Roche’s expertise to help your lab obtain reliable genomic insights.
Leveraging the FoundationOne® comprehensive genomic profiling (CGP) secondary analysis platform and the AVENIO workflow, our kit is part of Roche’s broad portfolio that offers flexible solutions and support services to meet your research needs. So you can get deeper genomic insights about solid tumors right in your lab — and advance discovery.
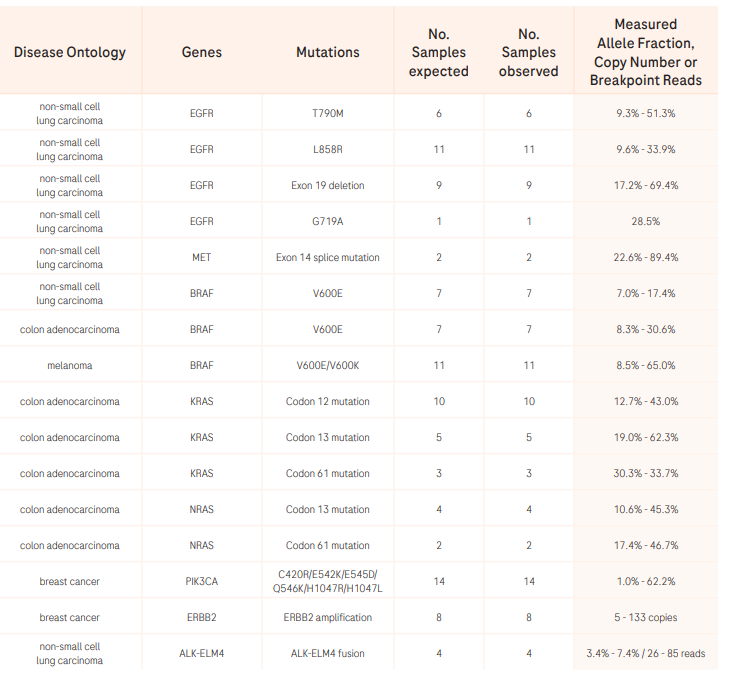
CGP offers the greatest insights from a single assay leveraging NGS to broadly analyze regions of the tumor genome that other tests miss.

References
| Material Number | Name | Price |
|---|---|---|
| 09523103001 | AVENIO Tumor Tissue CGP Kits - Plate A | Log in or register to see the price |
| 09523146001 | AVENIO Tumor Tissue CGP Kits - Plate B | Log in or register to see the price |


Material numbers, KK numbers, and product names
Connect your business to your Roche Sequencing Store account to use these additional features:
For privacy and security, your request will undergo manual review by Roche. Once approved, you'll receive an email. Your contracts and prices will then be accessible in the store.