The AVENIO Tumor Tissue Targeted Kit V2 is a next-generation sequencing (NGS) research assay for genomic profiling of solid tumors from formalin-fixed paraffin-embedded (FFPE) tissue samples. It contains 17 guideline-aligned biomarkers, including those in the U.S. National Comprehensive Cancer Network (NCCN) Guidelines.1
Features and Benefits
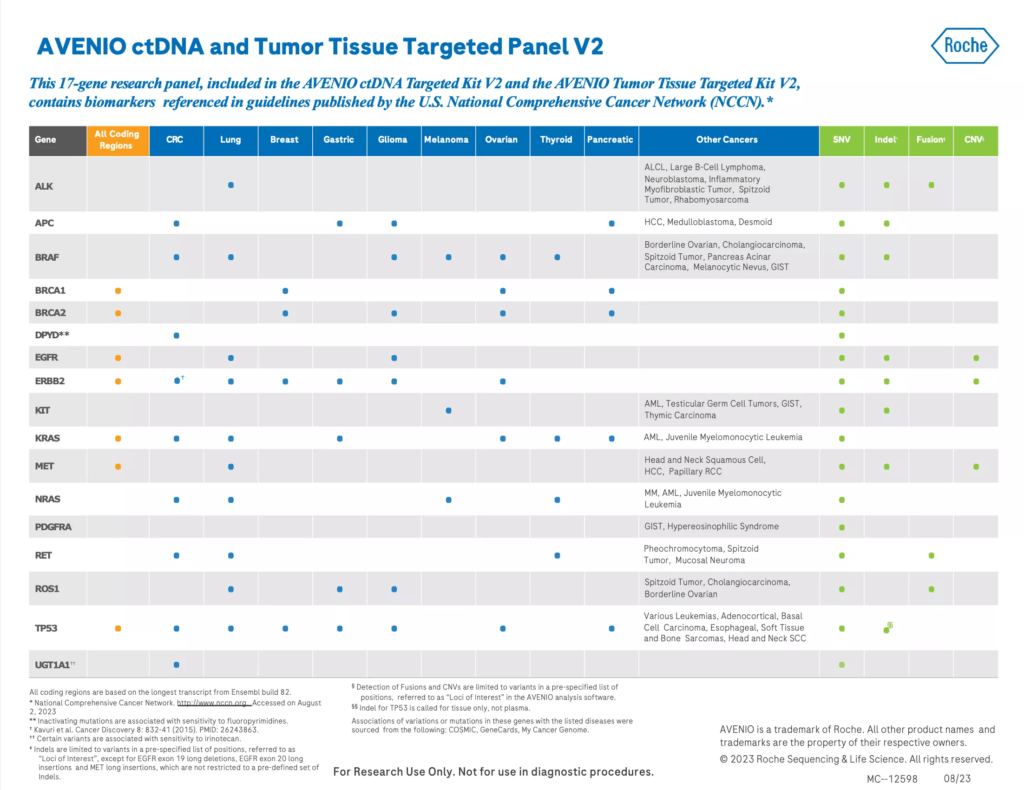
- Panel content exactly-matched to gene panel in the AVENIO ctDNA Targeted Kit V2 (same genes, gene regions and hybrid-capture workflow) to facilitate analytical concordance between tissue and plasma
- All four mutation classes (SNVs, indels, fusions and CNVs) in a single DNA workflow
- Flexibility to switch between tissue and plasma to support a variety of research applications
- A streamlined, end-to-end research workflow from extraction to analysis and reporting in 5 days
- A complete research solution that includes reagents, intuitive analysis and reporting to facilitate in-house adoption of high performance NGS oncology testing
Specifications
- Panel size: 81 kb
- Sample size: 2×10 µm FFPET curls/sections
- DNA input* 20 ng of amplifiable DNA
- Reactions per kit: 24
- Turn-around time: 5 days from extraction to results
* Total DNA amount for each sample was determined by input QC.
Contents
| Box # | Sub-kit name |
|---|---|
| 1 | AVENIO Tumor DNA Isolation and QC Kit |
| 2 | AVENIO Tumor Cleanup and Capture Beads V2 |
| 3 | AVENIO Tumor Library Prep Kit V2 |
| 4 | AVENIO Tumor Sample Primers – Plate A or Plate B |
| 5 | AVENIO Tumor Enrichment Kit V2 |
| 6 | AVENIO Tumor Tissue Targeted Panel V2 |
| 7 | AVENIO Post-Hybridization Kit V2 |
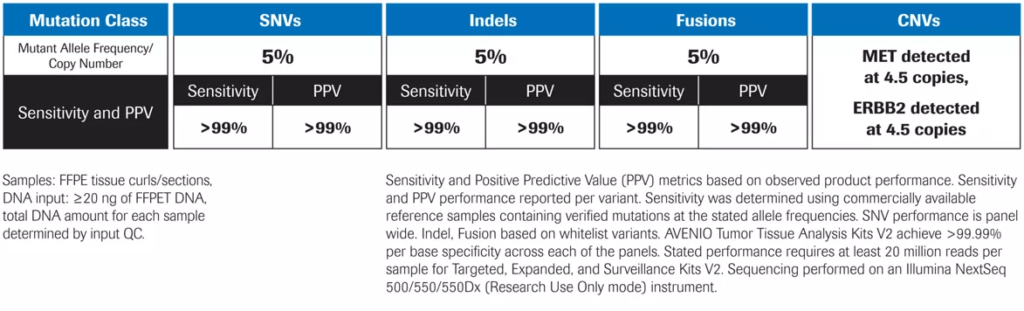
Exceptional Performance2
References
- National Comprehensive Cancer Network. Accessed Aug 2, 2023.
- Data on file with Roche.