Overview
Ready-to-use reaction mix containing FastStart Taq DNA Polymerase for hot start PCR, which significantly improves the specificity and sensitivity of PCR by minimizing the formation of nonspecific amplification products. The 2x master mix is optimized for a fixed MgCl2 concentration, which works with nearly all primer combinations. No adjustment in the MgCl2 concentration is needed to amplify different sequences; only template DNA, PCR primers, and hydrolysis probes must be added.
Highlights
- Save time with a convenient, ready-to-use 2x-concentrated hot start master mix.
- Perform sensitive and specific quantitative PCR, RT-PCR and endpoint genotyping analysis
- Eliminate time-consuming MgCl2 titration.
- Establish new qPCR assays, and get results quickly, by combining FastStart Essential Probes Master with Universal ProbeLibrary probes
How this product works
Sequence-specific detection of PCR products relies on sequence-specific oligonucleotide probes that are coupled to fluorophores. These probes hybridize to their complementary sequence in target PCR products.
Hydrolysis probe chemistry uses the so-called FRET principle. Fluorescence Resonance Energy Transfer (FRET) is based on the transfer of energy from one fluorophore (the donor or reporter) to another adjacent fluorophore (the acceptor or quencher).
Hydrolysis probe assays can technically be described as homogeneous 5′-nuclease assays, since a single 3′ non-extendable probe, which is cleaved during PCR amplification, is used to detect the accumulation of a specific target DNA sequence. This single probe contains two labels, a fluorescent reporter and a quencher, in close proximity to each other.
When the probe is intact, the quencher dye is close enough to the reporter dye to suppress the reporter fluorescent signal (fluorescent quenching takes place via FRET). During PCR, the 5′-nuclease activity of the polymerase cleaves the hydrolysis probe, separating the reporter and quencher. The reporter dye is no longer quenched and emits a fluorescent signal when excited.
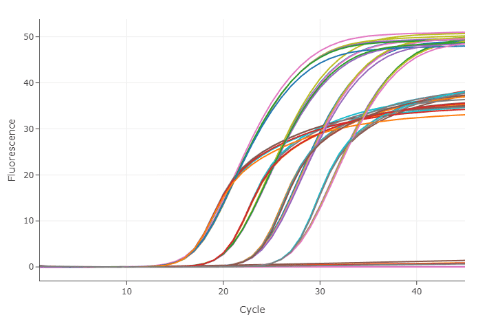
Figure: Duplex qPCR amplification reaction on the LightCycler ® PRO Instrument