The AVENIO Tumor Tissue CGP Kit V2 is an in-house next-generation sequencing (NGS) research assay that provides comprehensive genomic profiling from formalin-fixed paraffin-embedded (FFPE) tissue-derived DNA samples. With 335- gene panel aligned with the FoundationOne® CDx panel design and bioinformatics using the FoundationOne® Analysis Platform, the kit leverages the proven technology of Foundation Medicine and expertise of Roche to help your lab obtain reliable genomic insights.1-3
The power of precision medicine
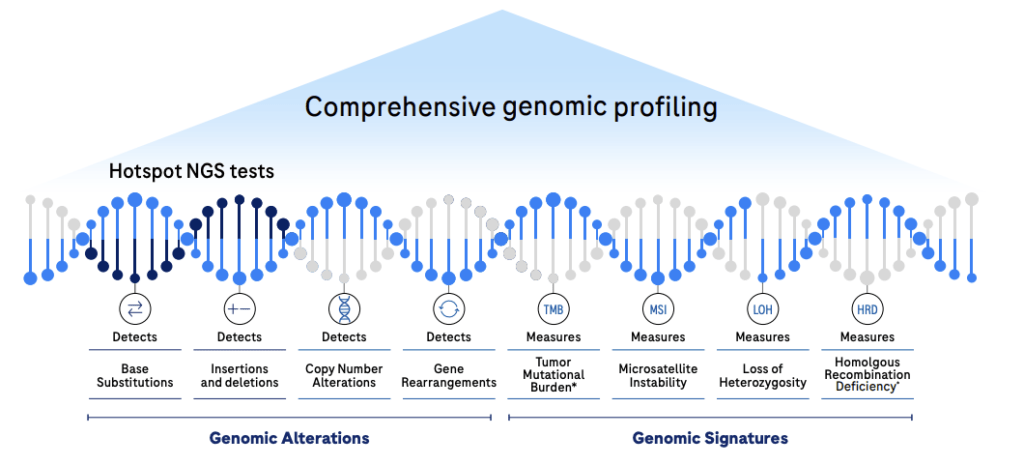
CGP offers the greatest insights from a single assay leveraging NGS to analyze broad regions of the tumor genome that are often missed by other targeted tests.6-16
Key assay attributes
Leverage the power of Roche and Foundation Medicine® – Experts in personalized medicine and comprehensive genomic profiling: 800+ peer-reviewed publications, 1.3 million+ clinical samples reported.1,4
Unlock high-quality meaningful genomic insights – Analyzes 335 relevant genes, four classes of genomic alterations, and complex genomic signatures including TMB, MSI, gLOH and the newly added HRDsig.2,3,5
Utilize fast and convenient NGS workflows – One workflow from DNA extraction to data analysis. Fast 2-day library preparation and short 1-hour ligation with a total 5-day turnaround time from DNA extraction to result generation.2,3
Robust analytical variant detection performance across genomic alterations and signatures
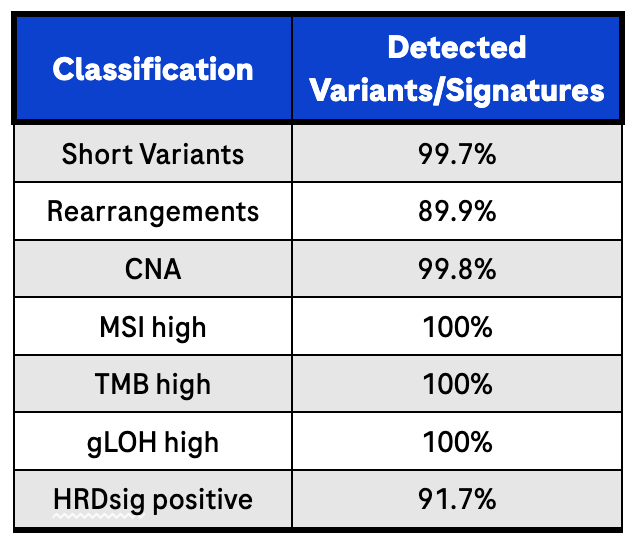
Sequencing libraries were prepared from 316 FFPE tissue- derived DNA using the Illumina NextSeq 550 High-throughput sequencing flowcell using the AVENIO Tumor Tissue CGP Kit V2.2
High performance as demonstrated by key sequencing metrics
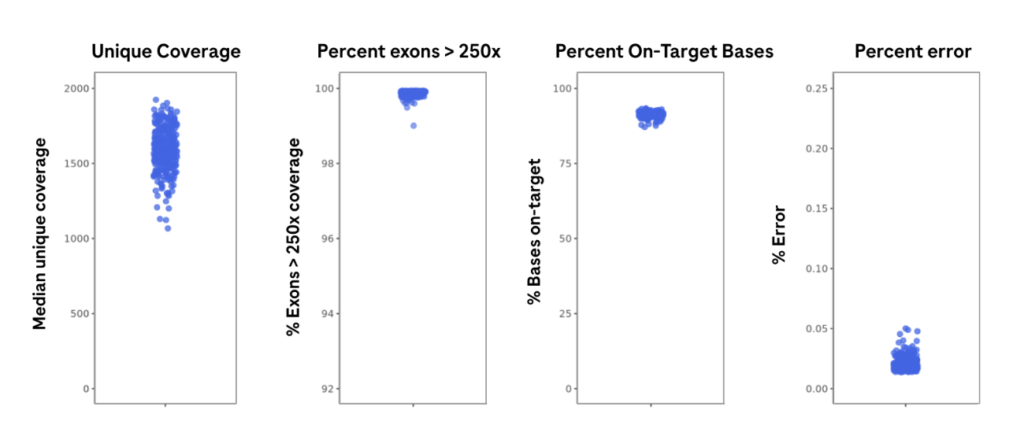
The graphs show sequencing QC metrics obtained through the FoundationOne® Analysis Platform. Results are from 60 million reads per sample (316 FFPE tissue- derived DNA samples) on Illumina NextSeq 550 (~12 samples per flowcell).2
Strong agreement in allele frequencies and signature scores to the reference method
Sequencing libraries were prepared from 316 FFPE tissue – derived DNA on the Illumina NextSeq 550 High-throughput sequencing flowcell using the AVENIO Tumor Tissue CGP V2 Kit. Results were compared with the analytical performance with the reference method and are shown.2
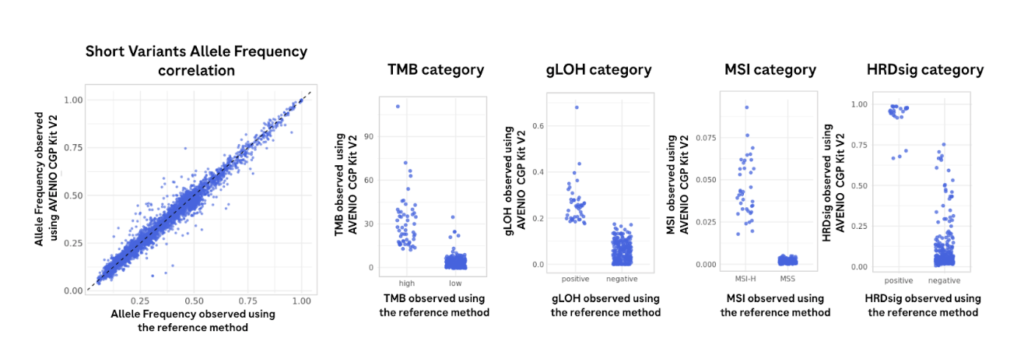
Results are from 60 million reads per sample on Illumina NextSeq 550 (~12 samples per flowcell) to determine performance by comparing to the reference method (FoundationOne®CDx).2 For this analysis, high or positive signatures were defined as follows: MSI-High ≥ 0.0124, TMB-High ≥ 10.0 mutations/Mb, gLOH-Positive ≥ 0.16, and HRDsig-Positive ≥ 0.7. Samples with scores in the marginal ranges, MSI (0.0041-0.0124; “equivocal” status), TMB (8.0-12.0 mutations/Mb), and gLOH (0.14-0.18), were excluded. The AVENIO Tumor Tissue CGP Kit V2 is a Research Use Only assay and should not be used for diagnostic procedures. Users must determine the complex signature cut-offs based on their research needs.
References
- Data on file with Roche.
- Choi et al. Evolution of a Comprehensive Genomic Profiling (CGP) Kit to Simplify Workflows and Detect Homologous Recombination Deficiency. Poster presented at Association of Molecular Pathology Europe, June 2024. https://medically.roche.com/global/en/oncology/amp-eu-2024/medical-material/AMP-EU-2024-poster-zhang-evolution-of-a-comprehensive-pdf.html.
- AVENIO Tumor Tissue CGP Kit V2 Instructions for Use June 2024.
- Foundation Medicine Biopharma services. Available at: https://www.foundationmedicine.com/info/biopharma-overview (Accessed July 2024).
- Chen KT et al. A Novel HRD Signature Is Predictive of FOLFIRINOX Benefit in Metastatic Pancreatic Cancer. Oncologist. 2023 Aug 3;28(8):691-698. doi: 10.1093/oncolo/oyad178
- Frampton GM et al. Nat Biotechnol. 2013;31:1023–1031.
- Suh JH et al. Oncologist. 2016;21:684–691.
- FoundationOne® CDx Technical Label, 2023. Available at: https://info.foundationmedicine.com/hubfs/FMI%20Labels/FoundationOne_CDx_Label_Technical_Info.pdf (Accessed July 2024).
- FoundationOne® Liquid Technical Specifications, 2023. Available at: https://assets.ctfassets.net/w98cd481qyp0/wVEm7VtICYR0sT5C1VbU7/fd055e0476183a6acd4eae6b583e3a00/F1LCDx_Technical_Specs_072021.pdf (Accessed July 2024).
- FoundationOne® Heme Technical Specifications, 2021. Available at: https://assets.ctfassets.net/w98cd481qyp0/42r1cTE8VR4137CaHrsaen/baf91080cb3d78a52ada10c6358fa130/FoundationOne_Heme_Technical_Specifications.pdf (Accessed July 2024).
- He J et al. Blood. 2016;127:3004–3014.
- Clark TA et al. J Mol Diagn. 2018;20:686–702.
- Chalmers ZR et al. Genome Med. 2017;9:34.
- Schrock AB et al. Clin Cancer Res. 2016;22:3281–3285.
- Ross JS et al. Gynecol Oncol. 2013;130:554–559.
- Hall MJ et al. J Clin Oncol. 2016;34:1523–1523.