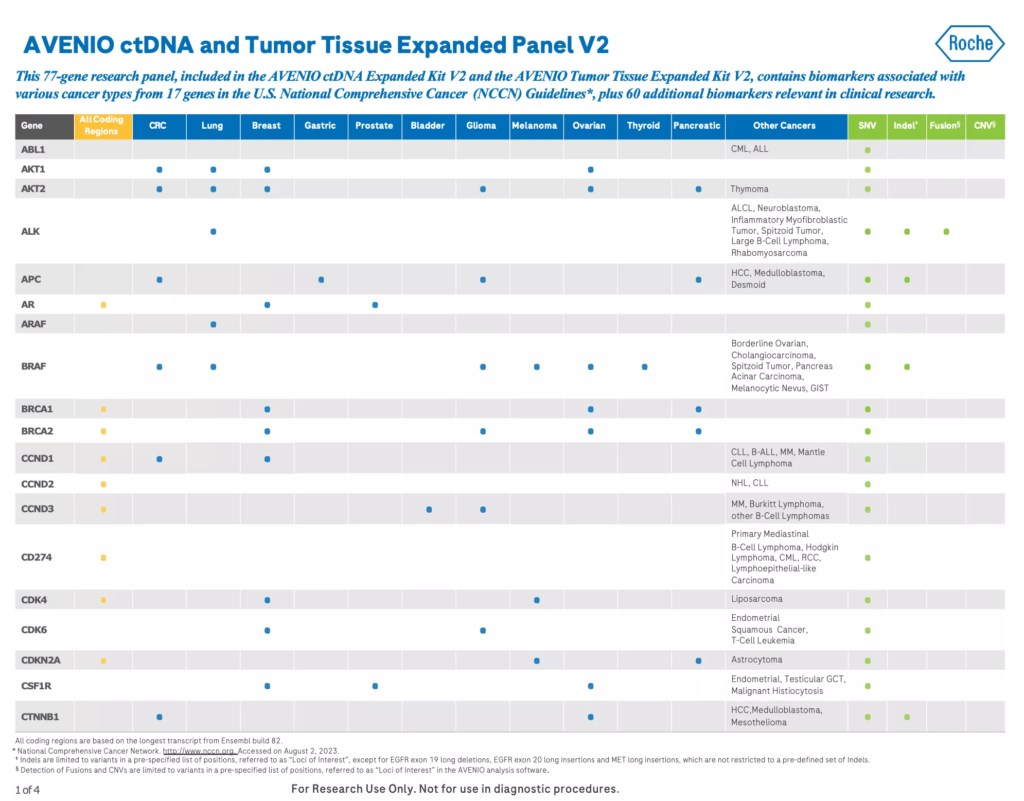
The AVENIO ctDNA Expanded Kit V2 is a next-generation sequencing (NGS) liquid biopsy research assay with a 77 gene panel containing genes in U.S. National Comprehensive Cancer Network (NCCN) Guidelines1 and emerging cancer biomarkers. It is a pan-cancer assay that’s specially optimized for lung cancer and colorectal cancer (CRC) research.
Features and Benefits
- Contains genes in the U.S. National Comprehensive Cancer Network (NCCN) Guidelines1 and emerging cancer biomarkers
- All four mutation classes (SNVs, indels, fusions and CNVs) in a single assay
- Exceptional analytical performance supported by integrated digital error suppression (iDES) strategies combining molecular barcodes with in silico error suppression techniques2,3
- Includes reagents, bioinformatics and software
- Streamlined, end-to-end research workflow from extraction to analysis and reporting in five days
Specifications
-
Panel size: 192 kb
-
Sample size: 4 ml of plasma
-
cfDNA input: 10-50 ng
-
Reactions per kit: 16
-
Turn-around time: 5 days from extraction to results
Contents
| Box # | Sub-kit name |
|---|---|
| 1 | AVENIO cfDNA Isolation Kit V2 |
| 2 | AVENIO Library Prep Kit V2 |
| 3 | AVENIO cfDNA Enrichment Kit V2 |
| 4 | AVENIO cfDNA Expanded Panel V2 |
| 5 | AVENIO Post-Hybridization Kit V2 |
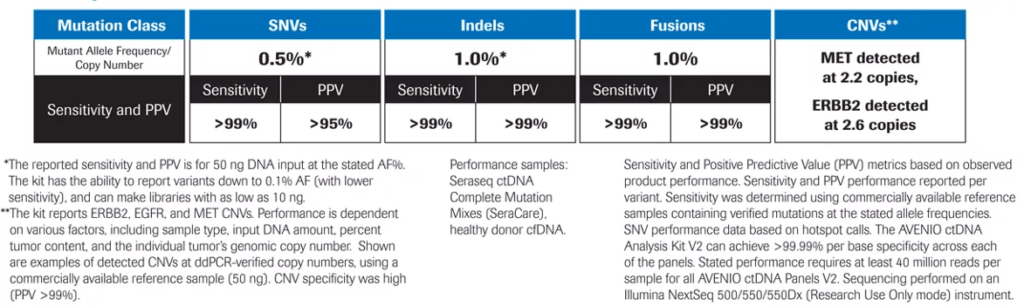
Exceptional Performance3
References
- National Comprehensive Cancer Network. Accessed August 2, 2023.
- An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature Medicine. 2014;20(5):548–554 doi:10.1038/nm.3519
- Data on file with Roche.