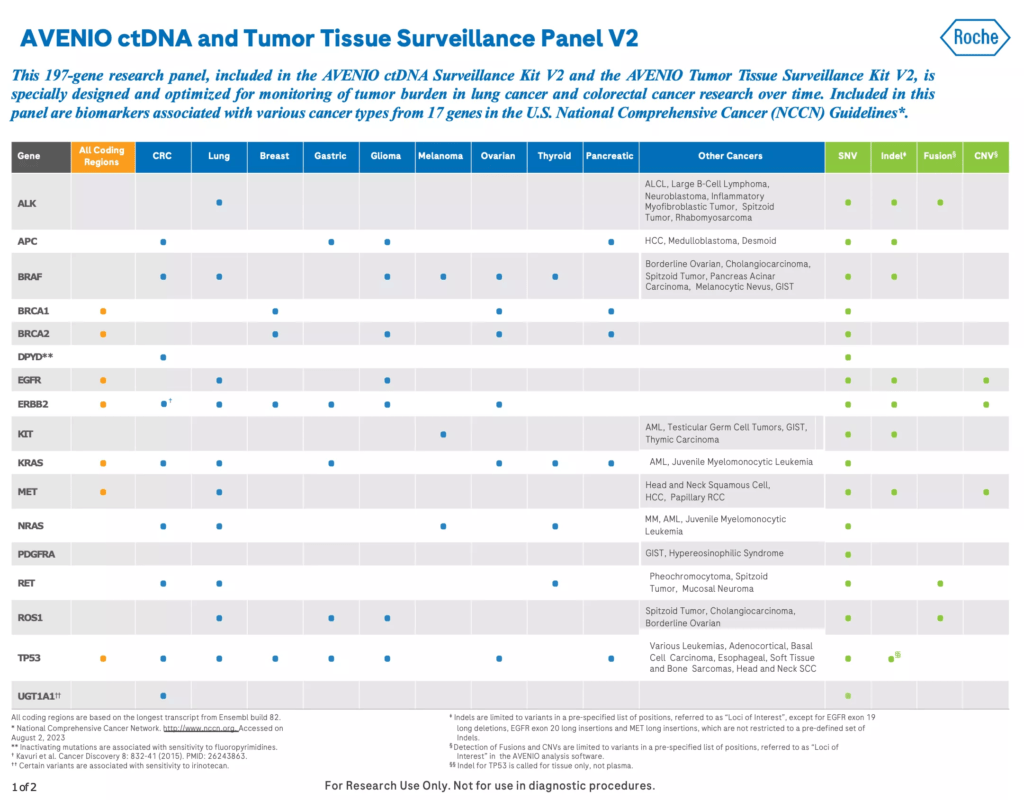
The AVENIO ctDNA Surveillance Kit is a next-generation sequencing (NGS) liquid biopsy research assay that’s specially designed and optimized for longitudinal tumor burden monitoring in lung cancer and colorectal cancer (CRC) research. The Surveillance panel contains 197 genes, including those in U.S. National Comprehensive Cancer Network (NCCN) Guidelines.1
Features and Benefits
- Includes genes in the U.S. National Comprehensive Cancer Network (NCCN) Guidelines1
- All four mutation classes (SNVs, indels, fusions and CNVs) in a single assay
- Exceptional analytical performance supported by integrated digital error suppression (iDES) strategies combining molecular barcodes with in silico error suppression techniques2,3
- Includes reagents, bioinformatics and software
- Streamlined, end-to-end research workflow from extraction to analysis and reporting in five days
Specifications
- Panel size: 198kb
- Sample size: 4 ml of plasma
- cfDNA input: 10-50 ng
- Reactions per kit: 16
- Turn-around time: 5 days from extraction to results
Contents
| Box # | Sub-kit name |
|---|---|
| 1 | AVENIO cfDNA Isolation Kit V2 |
| 2 | AVENIO Library Prep Kit V2 |
| 3 | AVENIO cfDNA Enrichment Kit V2 |
| 4 | AVENIO cfDNA Surveillance Panel V2 |
| 5 | AVENIO Post-Hybridization Kit V2 |
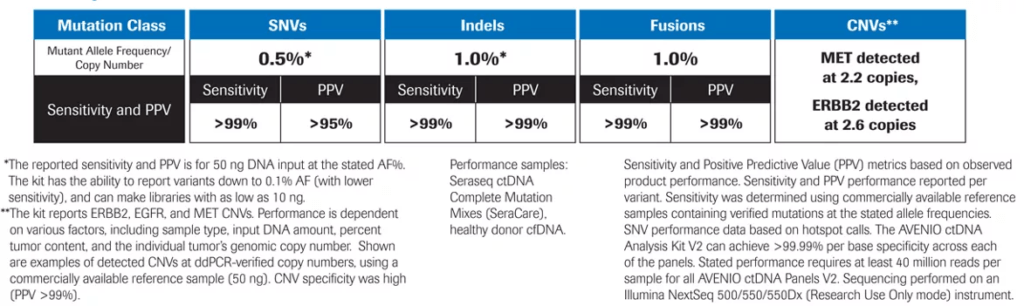
Exceptional Performance3
References
- National Comprehensive Cancer Network. Accessed August 2, 2023.
- Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature Medicine. 2014;20(5):548–554 doi:10.1038/nm.3519
- Data on file with Roche.




